|
A new genome tool has just become a bit easier to use in the lab with the publication of a knock-in CRISPR/Cas9 mouse. This tool allows researchers to easily edit the genome in specific locations so they can study the effect of a gene knockdown or alteration. Already, researchers were able to use this technique in vitro but now, a mouse strain has been made that can take this CRISPR/Cas9 system to the next level. CRISPR/Cas9 system CRISPRs (clustered regularly interspaced short palindromic repeats) are DNA loci containing short repetitions of base sequences. Each repetition is followed by short segments of "spacer DNA" from previous exposures to a virus. CRISPRs are found in approximately 40% of sequenced eubacteria genomes and 90% of sequenced archaea. CRISPRs are often associated with cas genes that code for proteins related to CRISPRs. The CRISPR/Cas system is a prokaryotic immune system that confers resistance to foreign genetic elements such as plasmids and phages[5][6] and provides a form of acquired immunity. CRISPR spacers recognize and cut these exogenous genetic elements in a manner analogous to RNAi in eukaryotic organisms. Since 2013, the CRISPR/Cas system has been used for gene editing (adding, disrupting or changing the sequence of specific genes) and gene regulation in species throughout the tree of life.[7] By delivering the Cas9 protein and appropriate guide RNAs into a cell, the organism's genome can be cut at any desired location. It may be possible to use CRISPR to build RNA-guided gene drives capable of altering the genomes of entire populations.[8] (Source: wikipedia.org) 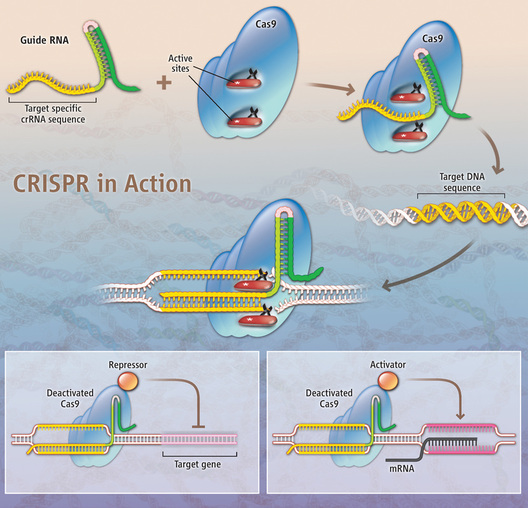 CREDIT: K. SUTLIFF/SCIENCE; SOURCE: Pennisi, E., 2013. The CRISPR Craze. Science 341, 833–836. With just a guide RNA and a protein called Cas9, researchers first showed that the CRISPR system can home in on and cut specific DNA, knocking out a gene or enabling part of it to be replaced by substitute DNA. More recently, Cas9 modifications have made possible the repression (lower left) or activation (lower right) of specific genes.  Previous issues with CRISPR/Cas9 The most important step in targeted genome editing is the creation of a double-stranded break in the DNA in the location that needs editing. These breaks in the DNA will trigger the repair machinery which will initiate one of the two DNA damage repair pathways: nonhomologous end-joining (NJH), resulting in insertions and deletions (indels), or homology-directed repair (HDR), resulting in point mutations or in precise sequence substitutions in the presence of a repair template. The CRISPR/Cas9 system requires two elements to do its genome editing work: Cas9, the DNA cutting enzyme, and guide RNA, to direct the Cas9 to the DNA sequence that needs to be cut. Cas9 can be easily reprogrammed using RNA guides to generate targeted DNA double-strand breaks, but until now, it was always required to provide the Cas9 protein along with the guide RNA to be able to edit a certain part of the genome. To get the Cas9 protein and guide RNAs into a cell, different vector delivery systems are used where the Cas9 gene sequence and guide RNA sequences are in tandem, but the inclusion of the Cas9 gene resulted in large delivery vectors. This made CRISPR editing especially difficult for applications in vivo, and consequently most CRISPR studies have been done in cultured cell lines. Cas9 knockin mouse This problem was recently overcome with the publication of a Cas9 knockin mouse strain by the groups of Phillip Sharp and Feng Zhang (Platt et al., 2014). In this mouse strain, the Cas9 gene is present in the mouse genome behind an inducible promoter and can be turned on when required, leaving a lot of room in the delivery vectors for more complex guide RNAs. After all, the Cas9 gene doesn't have to be included in the delivery vectors any more. The researchers showed that indeed it was possible to edit the genome in three separate genes with just one vector, thereby simultaneously creating loss-of-function mutations in two genes and a homology-directed-repair in another gene. In this way the authors could model the dynamics of the top three significantly mutated genes in lung adenocarcinoma in vivo. This makes the system particularly useful for cancer and other disease modelling as seldom only one mutation is the cause of a cancer or other disease. Transgenic Cas9 flies This was not the first report of a transgenic Cas9 animal, as a transgenic Drosophila strain was created a couple of months earlier, making in vivo gene studies in the fruit fly a lot more efficient. The CRISPR madness is not over yet, I think it is just starting! Reading:
1 Comment
7/3/2024 19:10:39
resulting in point mutations or in precise sequence substitutions in the presence of a repair template? Regard <a href="https://smb.telkomuniversity.ac.id/cerita-telutizen/pendaftaran-snbt-2024-resmi-dibuka-ini-link-dan-cara-daftarnya-jangan-sampai-ketinggalan/">Telkom University</a>
Reply
Leave a Reply. |
Archives
September 2018
Categories
All
|
 RSS Feed
RSS Feed






